Process Synthesis
The Process Synthesis Package contains several powerful tools for process development:
- prediction of azeotropic points in multicomponent mixtures
- construction of residual and boundary curves for ordinary distillation
- construction of contour lines
- entrainer selection for extractive and azeotropic distillation and extraction
It requires additional licenses for the DDB Access Package, the Mixture Predict Add-On as well as the data banks DDB-ACT and DDB-AZD for optimum performance.
Azeotropic Points in Multicomponent Mixtures
A first step in Process Synthesis for separation processes usually is the identification of azeotropic points in the multi-component mixture. The software is able to find homogeneous azeotropes in five component mixtures and heterogeneous azeotropes of up to three components. It utilizes the Wilson, NRTL and UNIQUAC model plus different group contribution methods like UNIFAC and mod. UNIFAC.
As an example the results for the quaternary system benzene (1) - cyclohexane (2) - acetone (3) - ethanol (4) at 1 atm are shown below together with experimental data from the DDB:

In process simulation, it is of great importance to verify, that the thermodynamic model and parameters correctly describe this hehavior!
Residual and Boundary Curves
Residual curves describe the course of a batch distillation. In case of a continuous rectification (no side streams, total reflux, ...) top and bottom product compositions are both situated on one residual curve. The feed composition can be found on the straight line connecting top and bottom product composition and can be calculated from the relative amounts of the products via mass balance.
Certain points in the ternary diagram, the pure components and azeotropic points, are of special importance. These points may be connected by boundary curves, which cannot easily be crossed by normal distillation, thus separating individual distillation fields. For homogeneous systems, feed and product compositions are situated in one distillation field. In case of heterogeneous systems, the liquid on a tray or in the condenser or reboiler may split into two phases. One of these phases or both may be situated in a different distillation field. This allows to cross distillation boundary lines by separating the two phases with the help of a decanter.
A ternary system without any azeotropic points does not contain any boundary lines and the residual curves connect the highest boiling component with the lowest boiling component. No residual curve originates or terminates in the medium boiling component. The pure medium boiling component represents a saddle point with respect to temperature or pressure. If any azeotropic points are present in the mixture, boundary lines can be found interconnecting the azeotropic and pure component points or additional azeotropic points. The areas inside the distillation region never show any maxima, minima or saddle points with respect to temperature or pressure. These extreme values or saddle points are always at the special points (pure components or azeotropic points).
The following plot shows the residual curve map for a complex ternary mixture with immiscible region calculated by the software. Three different feed concentrations and the resp. possible bottom (B) and distillate (D) concentrations have been added.
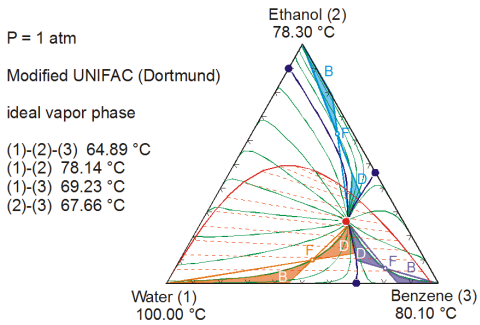
It is obvious that without the knowledge of this behavior, the design of a distillation column for this system is extremely difficult. In process simulation, it is of great importance to verify, that the thermodynamic model and parameters correctly describe this hehavior!
Contour Lines
Another possibility to show the behavior of ternary systems in a 2-dimensional form is with the help of the so-called contour or iso-lines. These curves represent all compositions in the ternary diagram, which show the same value for a selected property (e.g. boiling temperature or pressure, separation factor between two components, ...). Usually contour lines for different equally distanced values are included in one diagram.
Contour lines are of esp. importance to judge the effect of an entrainer on the separation of the two key components at varying entrainer concentrations. As an example, different lines at constant separation factor between benzene and cyclohexane are shown for the mixture with the common entrainer NMP at 1 atm in the figure below:

The mod. UNIFAC model predicts the behavior very close to the experimental finding with a monotonous decrease in separation factor to values below 0.4 with increasing entrainer concentration. Using UNIQUAC with parameters supplied by a major process simulation software leads to the unrealistic opposite effect at higher NMP concentrations.
This again stresses the great importance to verify the calculation results of the simulation thermodynamics!
Entrainer Selection
Due to the different advantages of distillation, this separation process is also employed in case of unfavorable separation factors. Different alternatives are available if ordinary distillation is not feasible:
- In some cases the azeotropic behavior disappears at lower or higher temperature (pressure) and ordinary distillation is possible at these different conditions.
- In case the azeotropic composition varies strongly with temperature (pressure), pressure swing distillation is often used. This situation arises mostly if the enthalpy of vaporization and consequently the slope of the vapor pressure curve of the two components to be separated differ significantly as for water plus organic components.
- In case the mixture forms a light boiling heterogeneous azeotrope, the distillate streams of two columns are condensed and the liquid phases are separated in a decanter and recycled to the columns. The pure compounds are obtained as the bottom products of the columns.
If neither of the above options solves the problem, separation can often be achieved by using an entrainer:
- In extractive distillation, a high boiling solvent is added slightly below the top of the column. Its task is to alter the ratio of the activity coefficients of the two components to be separated. In case of the aliphatic-aromatic separation, the interaction between e.g. NMP and the aromatic electrons of benzene lowers the volatility of benzene. At sufficiently high NMP concentrations this results in a separation factor of less than 0.4 between benzene and cyclohexane. In a second column the selective solvent is recovered and recycled to the extractor column.
- In case of azeotropic distillation, a suitable solvent is added which forms the lowest boiling azeotrope with one or both of the components to be separated. This light boiling azeotrope is obtained as the top product. In case of an hetero-azeotrope, separation of entrainer and product is usually simple. In other cases a similarly simple separation of this mixture has to be found.
A simple criterion to test the applicability of a component as extractive solvent is to calculate the activity coefficients at infinite dilution of the components to be separated in the entrainer. These should be as different as possible but high values of one of the activity coefficients (above 8 or 10) can lead to limited miscibility and therefore lower the capacity of the solvent. Potential entrainers for azeotropic distillation can be found by examining available data on binary or ternary azeotropes or by estimating these data using predictive models. In order to assist the engineer to find optimal entrainer candidates, a special program as part of the package “DDB Process Synthesis” has been developed. The software first examines the behavior of the binary mixture that needs to be separated and verifies the existence of an azeotrope. It will then analyze if this separation problem can be solved without introducing a further component (at reduced or elevated pressure, hetero-azeotropic distillation or pressure-swing distillation). If this is not possible, the program proceeds by searching for a suitable entrainer by direct access to the DDB (> 50 000 g∞ values, > 50 000 azeotropic or zeotropic data) or results from predictive methods for a large number of potential solvents (up to 25 000. The detailed procedure is shown below for both options:

Scientific Papers
| 2009 | Influence of ionic liquids on the separation factor of three standard separation problems | Westerholt A., Liebert V., Gmehling J. | Journal | Fluid Phase Equilib., 280, 1-2, 56 60 (2009) |
| 2005 | Recent Advances in Thermal Separation Processes | Steinigeweg S., Gmehling J. | Journal | Bunri Gijutsu, 35, 4, 214 222 (2005) |
| 2004 | Simple Method for Determining the Location of Distillation Region Boundaries in Quaternary Systems | Poepken T., Gmehling J. | Journal | Ind.Eng.Chem.Res., 43, 3, 777 783 (2004) |
| 2003 | Potential of Thermodynamic Tools (Group Contribution Methods, Factual Data Banks) for the Development of Chemical Processes | Gmehling J. | Journal | Fluid Phase Equilib., 210, 2, 161 173 (2003) |
| 2001 | Classification of Homogeneous Binary Azeotropes | Shulgin I., Fischer K., Noll O., Gmehling J. | Journal | Ind.Eng.Chem.Res., 40, 12, 2742 2747 (2001) |
| 1998 | Synthesis of Distillation Processes Using Thermodynamic Models and the Dortmund Data Bank | Gmehling J., Moellmann C. | Journal | Ind.Eng.Chem.Res., 37, 8, 3112 3123 (1998) |
| 1997 | Auswahl selektiver Zusatzstoffe fuer die Rektifikation durch kombinierten Zugriff auf experimentelle und vorausberechnete Gleichgewichtsdaten | Moellmann C., Gmehling J. | Journal | Chem.Ing.Tech., 69, 3, 324 328 (1997) |
| 1994 | Phasengleichgewichtsmodelle zur Synthese und Auslegung von Trennprozessen | Gmehling J. | Journal | Chem.Ing.Tech., 66, 6, 792 808 (1994) |
| 1993 | The Course of Distillation Curves in Ternary Systems | Novak J.P., Gmehling J., Matous J. | Journal | Chem.Listy, 87, 4, 754 775 (1993) |
| 1988 | Auswahl und Auslegung thermischer Trennprozesse mit Hilfe von Gruppenbeitragsmethoden | Gmehling J., Tiegs D., Weidlich U. | Journal | Chem.Ing.Tech., 60, 10, 759 768 (1988) |
| 1985 | Phasengleichgewichte als Basis zur Berechnung von thermischen Trennprozessen | Andereya E., Gmehling J., Heck G., Onken U. | Journal | Chem.Ing.Tech., 57, 2, 166 167 (1985) |
| 1985 | VLE Data for the Design of Separation Processes | Gmehling J. | Journal | AIChE Symp.Ser., 81, 244, 121 129 (1985) |
| 1979 | Auswahl von Loesungsmitteln fuer die Extraktiv-Rektifikation mittels vorrausberechneter Gleichgewichtsdaten | Kolbe B., Gmehling J., Onken U. | Journal | Ber.Bunsen-Ges.Phys.Chem., 83, 11, 1133 1136 (1979) |
| 1977 | Computerized Design of Multicomponent Distillation Columns Using the UNIFAC Group Contribution Method for Calculation of Activity Coefficients | Fredenslund A., Gmehling J., Michelsen M.L., Rasmussen P., Prausnitz J.M. | Journal | Ind.Eng.Chem. Process Des.Dev., 16, 4, 450 462 (1977) |


