LLE – Liquid-Liquid Equilibria

The LLE data bank contains a large amount of liquid-liquid equilibrium data and liquid solubility data for binary and higher systems. It is of great importance for extraction (distribution of a component between two liquid phases in equilibrium) and in case of other unit operations involving immiscible systems. The LLE data bank is required by the process synthesis software for the selection of solvents for liquid-liquid extraction using DDB.
Besides data for ternary and higher mixtures typically used for the design of extraction processes, the data bank contains a huge amount of information on mutual solubility as function of temperature and pressure. In case of low mutual solubility, activity coefficients at infinite dilution can directly be calculated from the solubility data.
Several different possibilities for the change of the immiscible region with temperature are given in the plot below together with the component combinations depicted in these examples. All data were taken from the DDB-LLE data bank.
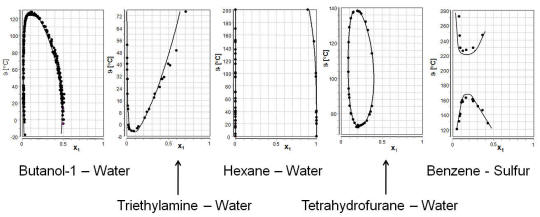
A full list of available systems is available in our DDB Online Search system. The page might take some seconds to load because of its size.
Data Tables, Monographies
| 1980 | DECHEMA Chemistry Data Series, Liquid-Liquid Equilibrium Data Collection. Part 2: Ternary Systems Part 3: Ternary and Quaternary Systems | Arlt W., Macedo M.E.A., Rasmussen P., Sorensen J.M. | Vol., 5.2+3, , 1-1290 (1980) |
| 1979 | DECHEMA Chemistry Data Series, Liquid-Liquid Equilibrium Data Collection. Part 1: Binary Systems | Arlt W., Macedo M.E.A., Rasmussen P., Sorensen J.M. | Vol., 5.1, , 1-650 (1979) |
Selected Scientific Papers
| 2005 | Extended Flexibility for GE Models and Simultaneous Description of Vapor-Liquid Equilibrium and Liquid-Liquid Equilibrium using a Nonlinear Transformation of the Concentration Dependence | Rarey J. | Journal | Ind.Eng.Chem.Res., 44, 19, 7600 7608 (2005) |
| 1998 | Separation of Enantiomers by Liquid-Liquid Counter Current Chromatography | Buchholz K., Schulte B., Gmehling J., Martens J. | Journal | Chem.Eng.Technol., 21, 5, 404 408 (1998) |
| 1991 | Darstellung der Temperaturabhaengigkeit binaerer Fluessig/Fluessig-Gleichgewichte mit Hilfe von gE-Modellen | Meyer T., Gmehling J. | Journal | Chem.Ing.Tech., 63, 5, 486 488 (1991) |
| 1991 | Correlation of binary liquid-liquid equilibrium data over a wide temperature range using UNIQUAC and extended UNIQUAC models | Nagata I., Meyer T., Gmehling J. | Journal | Fluid Phase Equilib., 65, 6, 19 39 (1991) |
| 1980 | Application of a Modified Wilson-Equation to Liquid Mixtures Showing Phase Splitting | Schulte H.W., Grenzheuser P., Gmehling J. | Journal | Fluid Phase Equilib., 4, 3, 185 196 (1980) |
Selected Scientific Papers (Experimental Data)
| 2007 | Vapor-Liquid Equilibria and HE for Binary Systems of Dimethyl Ether (DME) with C1-C4 Alkan-1-ols at 323.15 K and Liquid-Liquid Equilibria for Ternary System of DME + Methanol + Water at 313.15 K | Park S.-J., Han K.-J., Gmehling J. | Journal | J.Chem.Eng.Data, 52, 1, 230 234 (2007) |
| 2004 | Vapor-Liquid-Liquid Equilibria, Azeotropic, and Excess Enthalpy Data for the Binary System n-Undecane + Propionamide and Pure-Component Vapor Pressure and Density Data for Propionamide | Horstmann S., Fischer K., Gmehling J. | Journal | J.Chem.Eng.Data, 49, 6, 1494 1498 (2004) |
| 1994 | Vapor-Liquid and Liquid-Liquid Equilibria for the Toluene + 1,2-Propanediol + Water System | Fele L., Fermeglia M., Alessi P., Rarey J.R., Golob J. | Journal | J.Chem.Eng.Data, 39, 4, 735 741 (1994) |
Latest News
Links:


